Introduction to Clinical Trials
A clinical trial is defined as a prospective study comparing the effect or value of an intervention against a control in subjects. The core components of clinical trials are the population, intervention, control and outcome.
The steps in creating a clinical trial:
- Research question
- Design the trial (protocol)
- Enrollment/Data collection and check
- Conduct the trial
- Data freeze
- Data analysis
- Interpret results
Clinical Equipoise
The genuine uncertainty within the scientific and medical community as to which of the two interventions is superior.
FINER Criteria
- Feasible (is it possible?)
- Interesting
- Novel (is it new?)
- Ethical - always the most important
- Relevant (does it matter now?)
Belmont Principles
Relevant to the ethics of research involving human subjects:
- Respect of persons - informed consent
- Beneficence - to do no harm
- Justice - Fairness among those chosen for the study
Possible Objectives of the Intervention
The new experimental intervention being tested usually has one of the following primary objectives:
- Cure a disease
- Reduce disease symptoms
- Prevent disease worsening
- Prolong survival time (terminal disease) or prevent disease (vaccine)
- etc.
Types of Intervention Studies
- Pharmaceutical products
- Synthetic drugs
- Biologics
- Products made from human or animal cell/tissue such as vaccines or blood replacement products
- Medical Devices
- Pacemaker, cardiac stents, etc
- Other (education, exercise, therapy, etc)
Case-Control Experiments
Blinding ensures patients and/or investigators and/or analysts don't know which treatment is assigned to whom. It is not always possible to blind a study.
- Single-blind: One group does not know treatment assignment
- Double-blind: Two groups do not know treatment assignment (usually the subject and investigator)
- Open-label: Patient and investigators know the treatment
Placebo Control
A placebo is made of something that shouldn't have an effect on the body and is useful in determining if an intervention is "better than nothing". A placebo control theoretically has no physical effect on the disease, but it may have a psychological effect.
Active Control
Sometimes it is best to compare a new product to the currently marketed treatment or "standard of care". This would be preferable in control groups for terminal diseases, where it would be unethical to give a placebo.
Randomization
Allocation of the subject to one the interventions by chance. Yields the highest probability that treatments have a balanced distribution on measured and unmeasured covariates related to the outcome.
Non-Compliance in Clinical Trials
Some individuals never receive treatment to which they were randomized, stop treatment, or fail to consistently take treatment. We must consider how we approach this in our analysis:
- Intention to treat: Compare randomized groups regardless of compliance (usual primary approach)
- Modified intention to treat: Exclude patients who were randomized but not eligible or did not have any post-baseline data
- Per-protocol: Compare only individuals who took the medication and/or are compliant with the assigned treatment and were eligible
Study Designs
Parallel Group Design
Most common design and the main focus of this course. Multiple treatment groups and subjects are randomized to exactly 1 treatment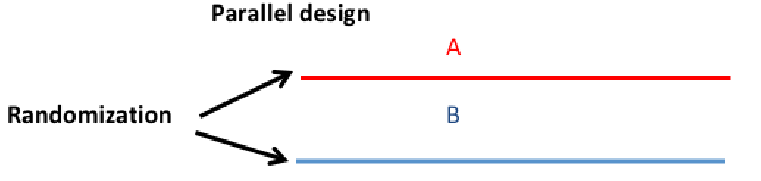
Crossover Design
Each patient is randomized to a random sequence of treatment that will be administered sequentially. The objective remains to compare treatment A vs B. Typically less individuals are required.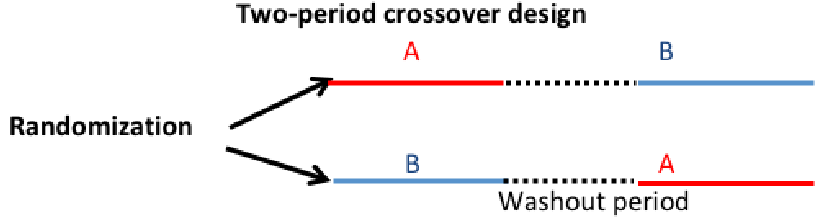
Factorial Design
Often researchers are interested in studying the effect of two or more interventions applied alone or in combination.
Ex. 2x2 factorial design in which two interventions in A and B are evaluated
| (left is A, top is B) |
- |
+ |
| - |
Control |
B only |
| + |
A only |
A and B |
Study Protocol
Every trial has a written protocol, documenting all information concerning the purpose, the design, conduct of the trial and data analysis. This promotes good faith by stating what data is collected and for what purpose, and facilitates communication with relevant ethics committee or regulatory bodies and the sponsor.
- Administrative information
- Title, trial registration, protocol version, funding, roles and responsibilities
- Introduction
- Background
- Research hypotheses
- Study design
- Methods
- Settings and recruitment
- Eligibility criteria
- Treatment/interventions
- Primary outcome
- Timeline
- Sample size conciderations
- Treatment allocation, randomization and blinding
- Data collection and data management
- Statistical analysis plan
- Data monitoring and auditing
- Ethics, ethics approval, protocol amendments, consent, confidentiality, declaration of interests, access to data, post-trial care and dissemination policy
Every clinical trial regardless of the sponsor must be registered at
